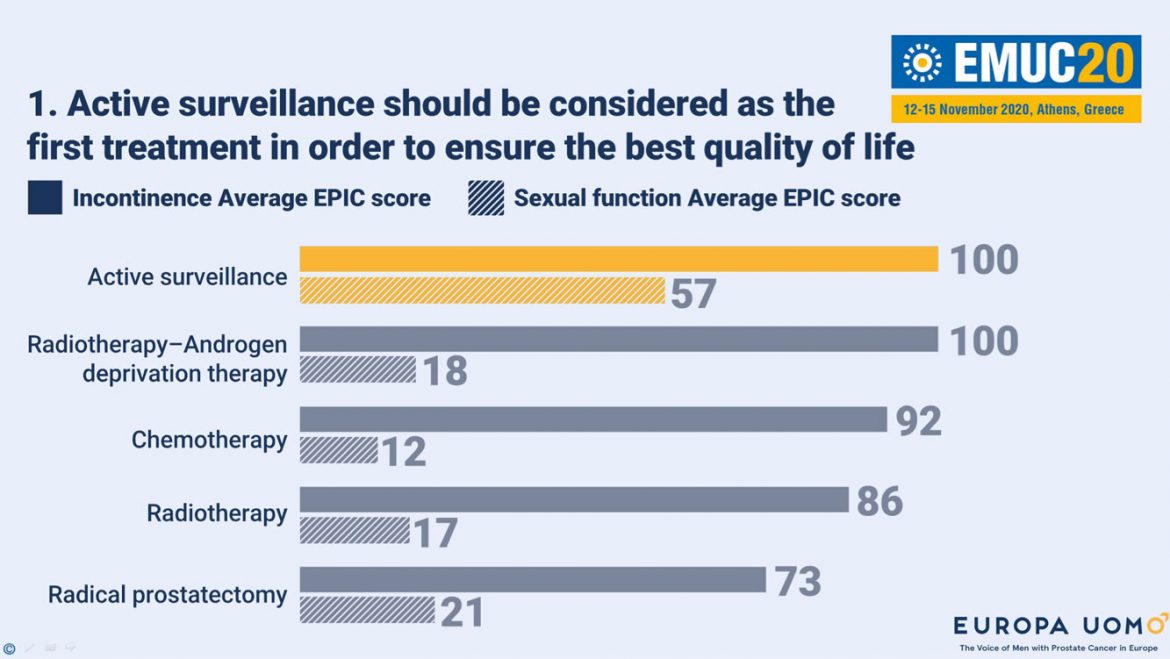
Active surveillance should be considered the first treatment for prostate cancer in order to ensure the best quality of life. This was one of the key messages of Mr. André Deschamps’ (BE) presentation during the “Highlights in GU cancers” session on the first day of the 12th European Multidisciplinary Congress on Urological Cancers (EMUC20 Virtual). Mr. Deschamps presented the results of the EUPROMS study, a Patient-Reported Outcomes (PRO) study conducted by the patient advocacy organisation Europa Uomo.
The EUPROMS results revealed that active surveillance had the best average Expanded Prostate Cancer Index Composite (EPIC) score in incontinence (100) and sexual function (57). Respondents, 2943 prostate cancer patients from 24 European countries who received treatment, also experienced less tiredness, insomnia, and pain or discomfort while under active surveillance in comparison with other treatments. This led to Mr. Deschamps’ logical conclusion: “When it can be applied safely, active surveillance should be considered the first treatment for prostate cancer.”
During the discussion, via the interactive feature of the congress platform, Mr. Deschamps received two questions about the difference between the EUPROMS study and a clinical study. “It is a different kind of survey that we did. We wanted to have a picture of what the real quality of life of men is after treatment,” he clarified. “I think that clinical studies should be taking the quality of life into account as well: as a primary endpoint, in fact. Of course it is also important to consider life extension when deciding to spend a lot of money on a new drug, but quality of life should always be an endpoint, too.”
In the same session, Prof. Jonathan Epstein (US) addressed the controversies and uncertainty that persist in prostate cancer grading and the first prostate cancer grading recommendations from the Genitourinary Pathology Society (GUPS) that focuses on these areas. Prof. Anders Bjartell (SE), chairman of the EAU Research Foundation (EAU RF), gave an update on the EAU RF trials in progress such as the PEGASUS study. He also touched on the PRECISION study, which became a game-changing trial showing the value of MRI before a prostate biopsy. The Nimbus study, which was stopped early due to safety reasons, was further discussed in the later session “New trials update.”
This session on GU cancer highlights was only one of the highlights of EMUC20 Virtual‘s first day. Covering a broad spectrum of topics in genitourinary malignancies, EMUC20 Virtual features speakers from all over the world. Unique about this first day was, characteristic of the year 2020, the roundtable about the impact of the Covid-19 epidemic on the management of GU cancers.
(Re)watch a session? All sessions are available on demand in the Resource Centre.